Fda Food Facility Registration Exemptions
Compliance policy guide guidance for fda staff.
Fda food facility registration exemptions. Mixed type facility means an establishment that engages in both activities that are exempt from registration under section 415 of the federal food drug and cosmetic act and activities that. This chart describes the definition. For the purposes of food facility registration contains nonbinding recommendations 26 the definition of food in 21 cfr 1 227 excludes food contact substances as defined in section 409 h 6 of the fd c act 21 u s c. Food facility registration seventh edition.
If you are an owner. The fda food safety modernization act fsma pub. Compliance policy guide sec. One of the exemptions is for retail food establishments rfes as defined in 21 cfr 1 227.
Unless the facility satisfies one of the exemptions in 21 cfr 1 226. 111 353 enacted on january 4 2011 amended the food facility registration requirements in section 415 of the fd c act. In addition to the secg fda also updated their registration of food facilities webpage to include a flowchart meant to help facilities determine if they are considered a retail food establishment. Unlike the registration requirements for drugs and devices that result in misbranding violations under 502 o of the fd c act the failure of a food facility to be registered with fda does not.
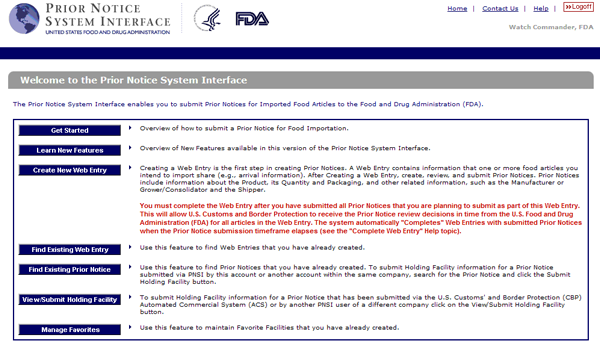
Please note that facilities must have a valid food facility. You must register with fda unless you are exempt under 21 cfr 1 226 from the requirement to register. The two forms form fda 3942a for human food or form fda 3942b for animal food should be used to submit attestations.