Extraction Of Caffeine From Tea Lab Report Conclusion

An acid base liquid liquid extraction took place in order to force caffeine into the organic layer.
Extraction of caffeine from tea lab report conclusion. Distill the extraction solvent 1 propanol from the caffeine. The percentage yield of caffeine is 89 98. In the liquid liquid dcm water. The tea is made under basic conditions to ionize mildly acidic flavonoids and tannins causing them to be more water soluble.
To extract caffeine from tea 2. The melting point of caffeine that was obtained in this experiment was 224 c. Organic chemistry lab labs extraction of caffeine from tea leaves background how to use leftover green tea leaves68291dd53955f34d9d7a jpg 56 4 kb the active ingredient that makes tea and coffee valuable to humans is caffeine. 2014 extracted the amount of caffeine from used tea leaves of black white green and red tea using dichloromethane as solvent and found that caffeine content was maximum 60 mg.
The amount of caffeine in a tea bag is 0 0979 g. Since caffeine is more soluble in dichloromethane 140 mg ml than it is in water 22 mg ml it readily dissolves in the dichloromethane. Take 500 ml beaker add 200 ml of distilled water to it. This gave calculated values of 59 1 recovery and 40 9 error.
Size of the plant tea leaves contain 1 4 to 4 5 caffeine by weight the world s most used drug step 13. The melting point of caffeine is 228 6. In this experiment the caffeine was successfully extracted from a 2 26g tea bag was 0 008g and the percentage recovered of caffeine in the tea bag was 0 35. To purify caffeine by recrystallization 5.
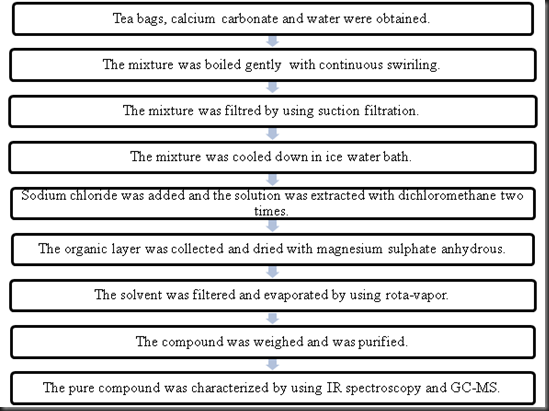
Conclusion in this experiment the caffeine was extracted from the tea bag. The hot solution is allowed to cool and the caffeine is then extracted from the water with dichloromethane methylene chloride which is an organic solvent that is insoluble in water. To perform a vacuum filtration 3. The caffeine was evaporated and appeared in yellowish form.
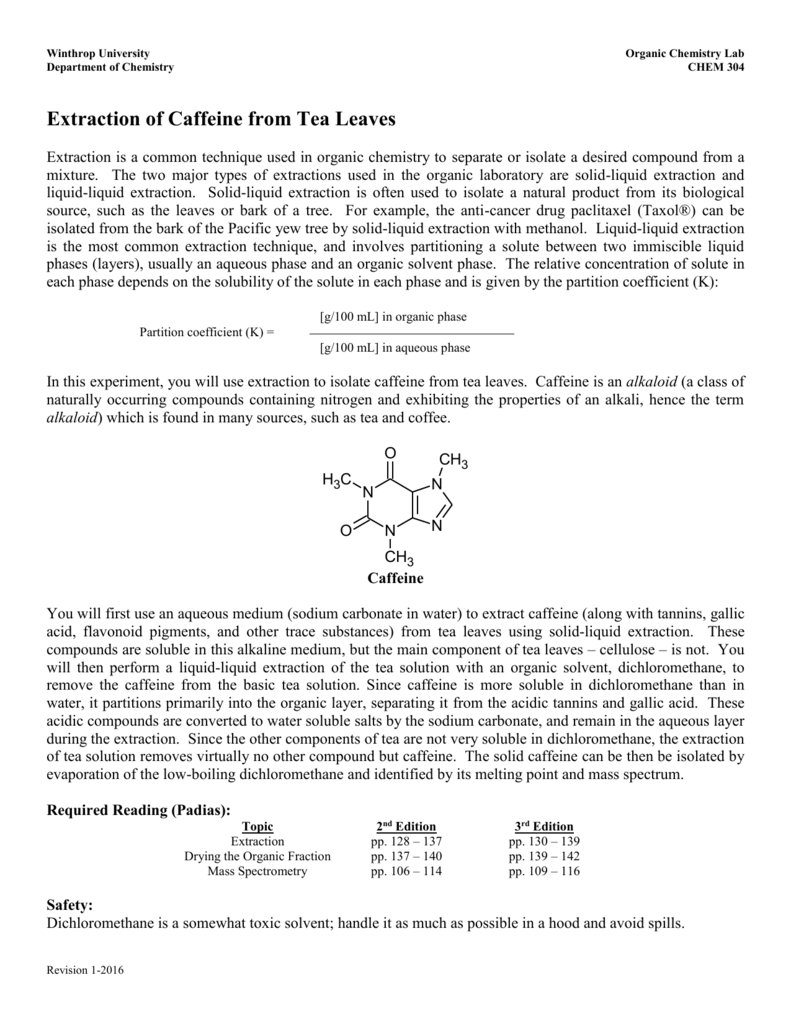
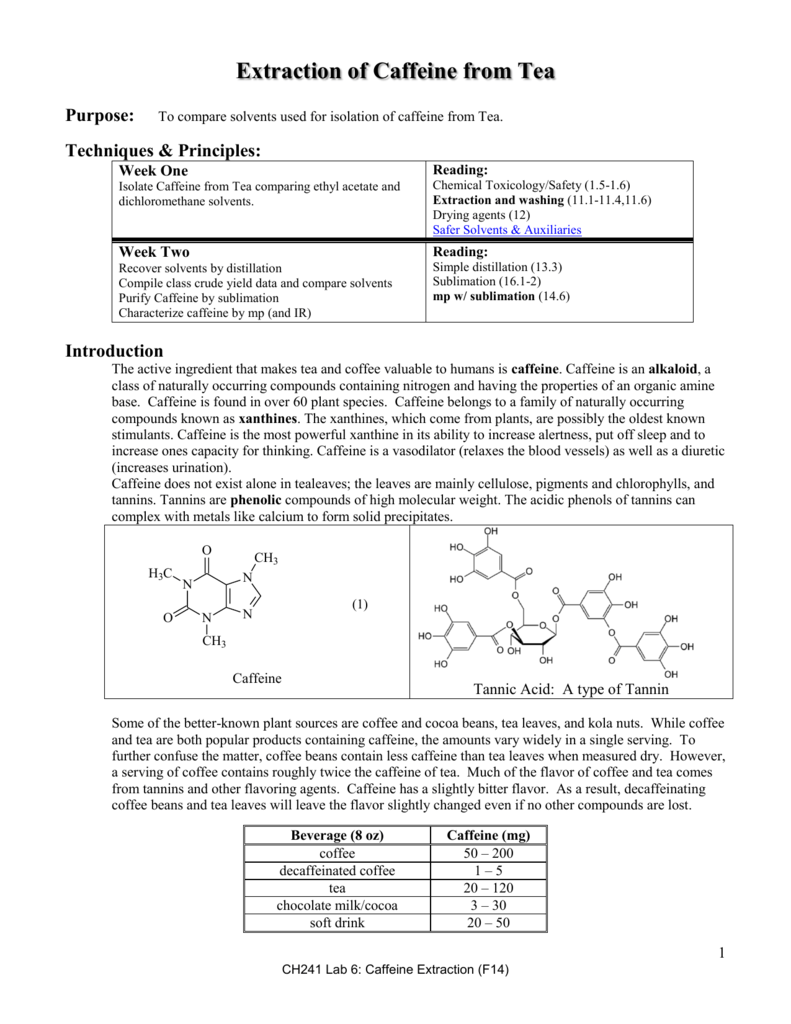
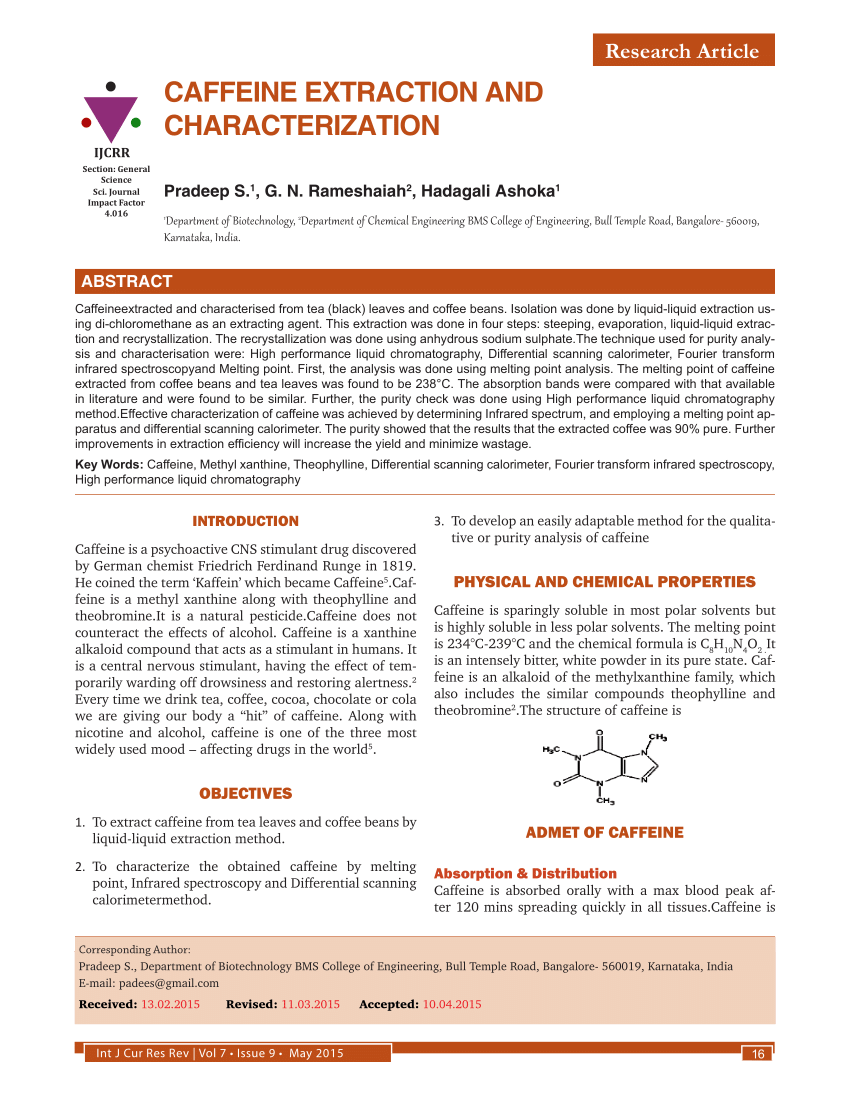
To determine the percent of caffeine per gram of instant tea descriptions caffeine is a natural product belonging to the alkaloid group. Caffeine is in one gram of a typical caffeinated beverage such as instant tea. Caffeine was extracted from tea by the use of solid liquid and liquid liquid extractions. A pure product of 065 g caffeine was obtained.
Take 5 tea bags and record the weight of these tea bags. Tea is one of the most commonly used caffeinated beverages in the world. Now place the 5 tea bags in this beaker.