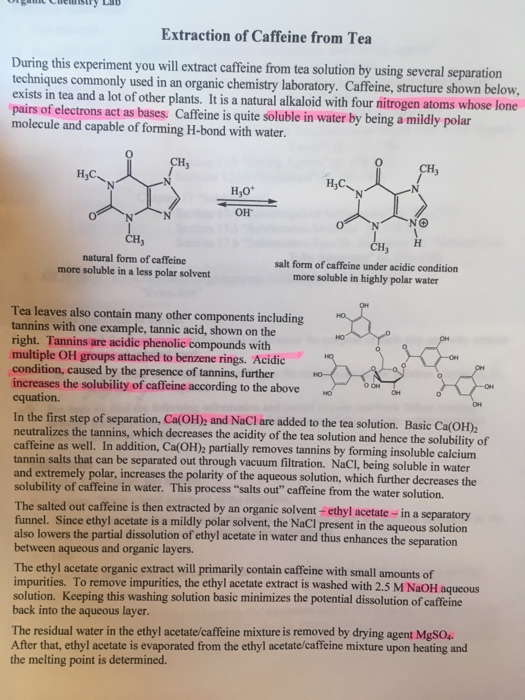
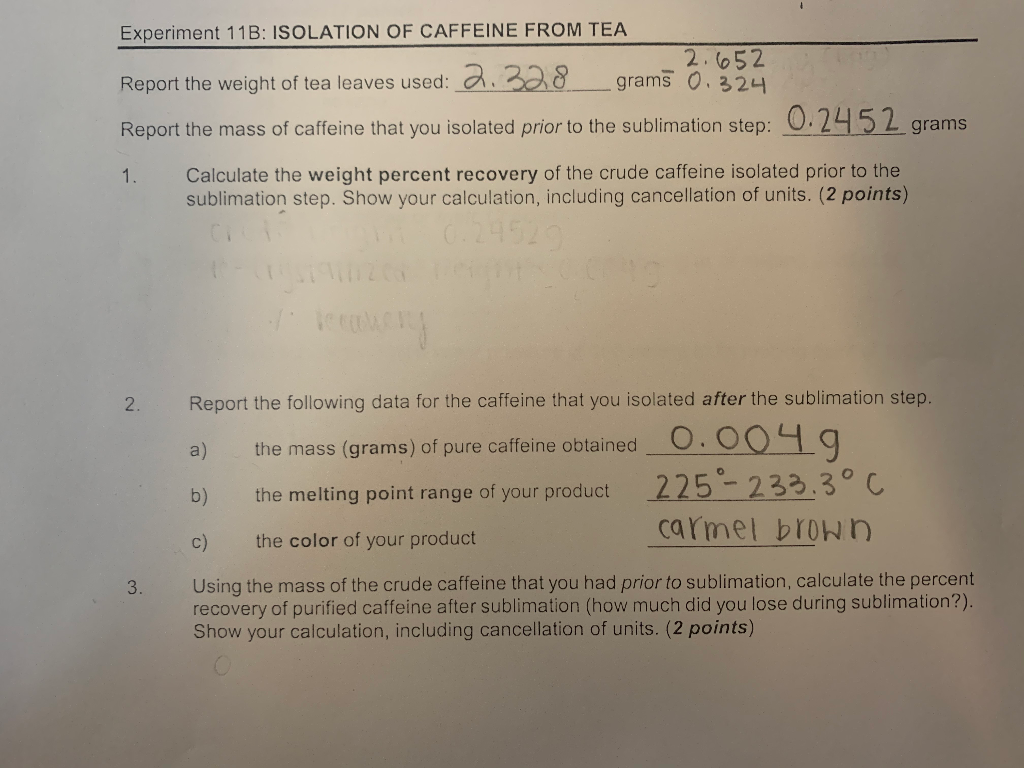
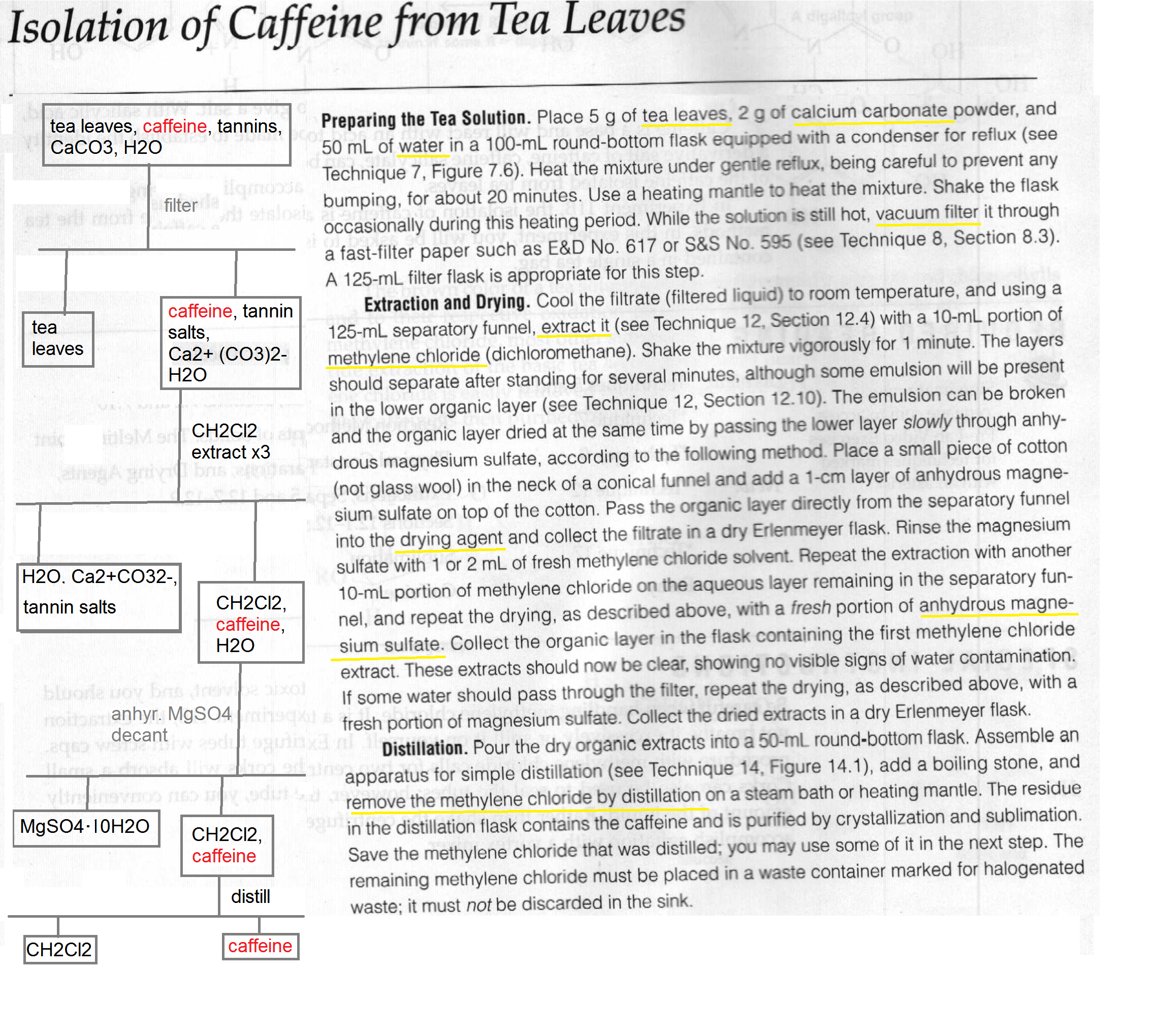
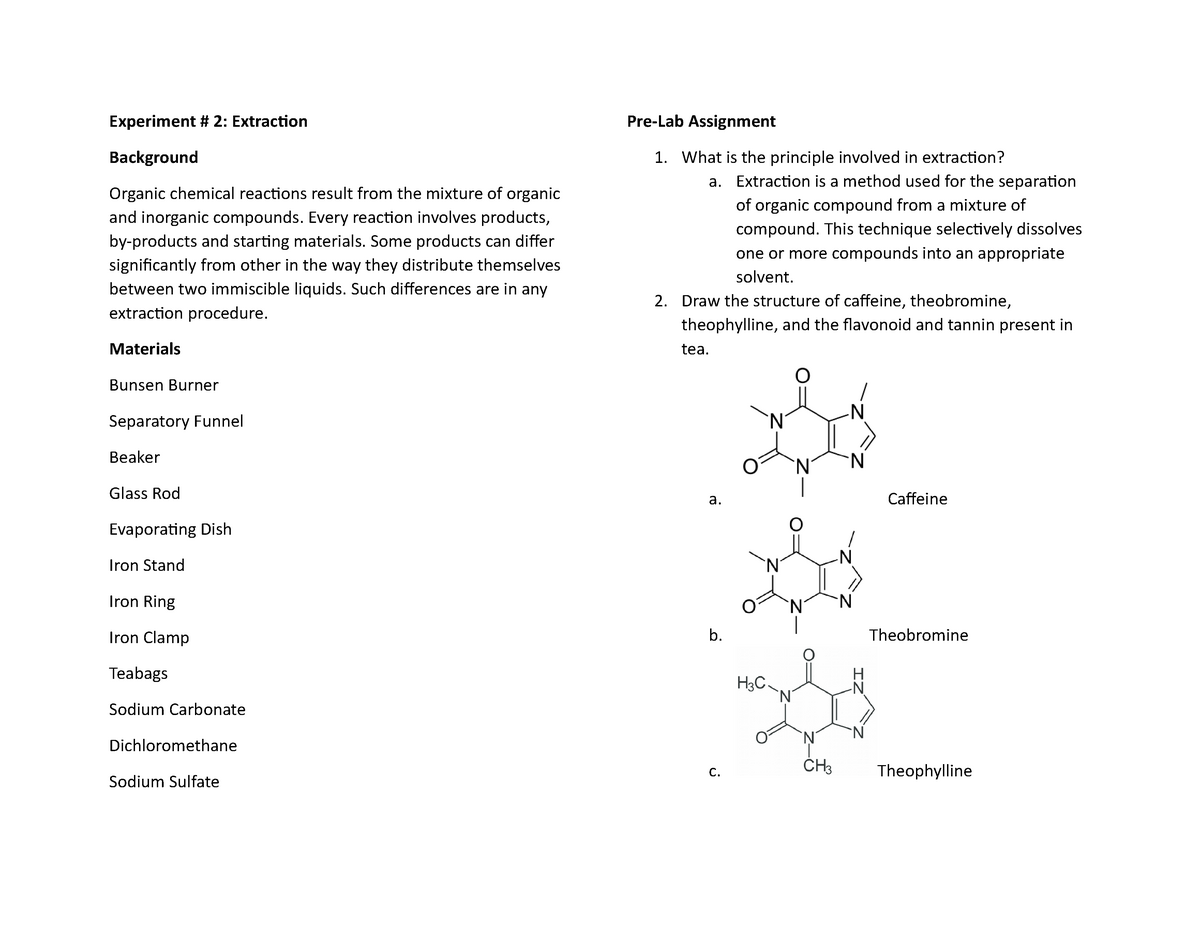
Extraction Of Caffeine From Tea Lab Report Answers

This gave calculated values of 59 1 recovery and 40 9 error.
Extraction of caffeine from tea lab report answers. Take 500 ml beaker add 200 ml of distilled water to it. Data and results caffeine extraction worksheet tea extract description data 1 volume of extract ml amount of caffeine in extract mg ml hplc sample 1 2 total caffeine in 100 ml sample 3 tare weight of rb flask 4 final weight of flask dried caffeine 5 crude caffeine isolated mg 4 3 6 yield of crude caffeine 5 2 100. Now place the 5 tea bags in this beaker. Caffeine was extracted from tea by the use of solid liquid and liquid liquid extractions.
Extracting caffeine from tea extracting caffeine from tea leaves research question. Use hot water to extract the caffeine from the tea leaves. A student was confronted with the problem of tannin contamination while extracting caffeine from tea leaves. I have a lab report on the extraction of caffeine from tea.
Tea bags are used as the source of caffeine for this experiment. Take 5 tea bags and record the weight of these tea bags. Those three molecules are caffeine tannis and glucose. What will happen if sodium sulfite is not added to the caffeine extract.
Another way to extract caffeine from tea is to brew tea in hot water allow it to cool to room temperature or below and add dichloromethane to the tea. While cellulose is insoluble in the water the tannins and chlorophyll will extract along with the caffeine into the water. You will get caffeine in the heavier dichloromethane layer. Table of information graph of weight extraction and purification method part 1.
The caffeine preferentially dissolves in dichloromethane so if you swirl the solution and allow the solvent layers to separate. The hot solution is allowed to cool and the caffeine is then extracted from the water with dichloromethane methylene chloride which is an organic solvent that is insoluble in water. However the molecules given above for the lab report template are not those of caffeine tannis and glucose. A pure product of 065 g caffeine was obtained.
Extraction of caffeine step 4. In this experiment the caffeine was successfully extracted from a 2 26g tea bag was 0 008g and the percentage recovered of caffeine in the tea bag was 0 35. Since caffeine is more soluble in dichloromethane 140 mg ml than it is in water 22 mg ml it readily dissolves in the dichloromethane. Tea is one of the most commonly used caffeinated beverages in the world.
Which type of tea leaves white green and black has the most amount of caffeine. This is extraction of caffeine from tea lab. After the first step solid liquid extraction we are left with three molecules to now separate. The solubility of caffeine is 22 mg ml 25 c and 670 mg ml 100 c.